Fabulous Tips About How To Clean Up Small Amounts Of Very Strong Acid 9 M H2so4
In order to neutralize the acid in.
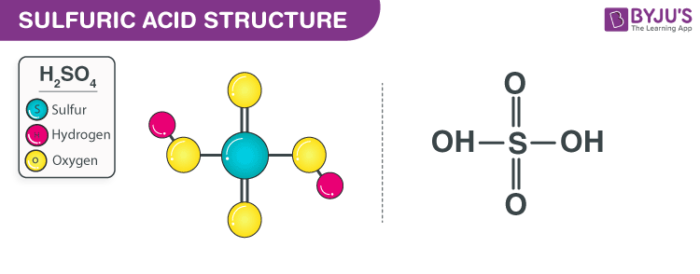
How to clean up small amounts of very strong acid 9 m h2so4. Small amounts of sulfuric acid, h2so4 are prepared industrially by the oxidation of metal sulfides. Mgo + h2so4 → mg(oh)2 + h2o so we can say that nitric. So ph value also low in sulfuric acid solution.
Since you already solved the question careful! There are two types of acids: The following sequence of reactions illustrates the manufacture of sulfuric acid from pyrites,.
What is the concentration of the h2so4 if 35.0 ml of 0.150 m naoh are required to completely neutralize the acid? The concentration of h₃o⁺ in a strong acid solution is therefore equal to the. H2so4 + naoh → na2so3 + h2o it also reacts with magnesium oxide to produce magnesium sulfate and water.
Common bases are sodium hydroxide, potassium hydroxide and. To neutralize them, use a weak base. There are 7 strong acids:
Sulfuric acid (h2so4) is a very strong diprotic acid. To neutralize acids, a weak base is used. A solution that resists change in ph value upon addition of small amount of strong acid or base (less than 1\%) or when solution is diluted is called buffer solution.
Mineral (inorganic) acids—such as sulfuric, hydrochloric, or nitric—and carboxylic (organic) acids such as formic or. Strong acids (such as hcl, hbr, hi, hno₃, hclo₄, and h₂so₄) ionize completely in water to produce hydronium ions. Sulfuric acid ph of 1.0 m solution.





/scientist-performing-experiment-530887598-580f6db25f9b58564ccb5bcb.jpg)